
6 of the Best in Structural Heart.
The structural heart space is one of the most exciting areas in the whole of medical devices. A broad term, covering a range of cardiac conditions, the advances in technology over the last 20 years has been extremely impressive, with no sign of slowing down.
As with any relatively new area of study, the structural heart space is full of start ups working on what they think may be the next big breakthrough in the market. I’ve been lucky enough to spend time speaking with leaders from six such companies, all of whom are pre-commercial but at differing stages in their development.
These companies span the globe and approach problems and conditions associated with structural heart diseases in a variety of ways. The one thing they have in common is that they are all forward thinking, exciting businesses that are in the process of bringing remarkable new solutions to the market.
NeoVasc – TIARA
Mitral regurgitation (MR) is the most common form of heart valve disease, with millions of patients suffering from the condition around the world. It’s caused by a faulty mitral valve and can have severe consequences for sufferers, sometimes resulting in death.
Neovasc’s TIARA is a replacement mitral valve which they hope will fill one of the biggest holes in the structural heart market. Emerging less-invasive approaches are only appropriate for around 20% of patients which leaves a large majority of patients left un-treatable.
COO Bill Little told me that, so far, they had treated 82 patients with their trans-apical offering, which has enabled them to learn a great deal about the implantation of the valve. They’ve also developed another version of TIARA, for trans-femoral application.
NeoVasc believe that the trans femoral version of TIARA could be the real game changer for the industry. The company hope to be able to treat upwards of 70% of eligible patients with this system when it’s ready to go, a massive development in coronary care.
NeoVasc and TIARA are slightly further along in their journey than some of the other companies in this list. With just less than 100 employees they’re hoping to file for CE Mark for the trans-apical implant this year and are just starting the first human implants with the trans-femoral version of TIARA.
As if that weren’t enough, they have also developed Neovasc Reducer, an angina treatment declared a breakthrough medical device by the FDA.
Append Medical - Appligator

Zachi Berger decided to go it alone at the end of 2016 after a career spent working across molecular biology and later with VC businesses as an investor. He came across the technology which became Append when looking for innovative new projects coming out of Sheba Medical Centre, Israel’s largest.
His first move was to license the tech from Sheba before partnering with Israeli incubator MEDX Xelerator which is backed by both the Sheba Medical Centre and Boston Scientific, as well as MEDX Ventures and Intellectual Ventures. This has had a huge impact on Append’s trajectory. It’s been quite the journey from interesting technological innovation to where they are at today.
Append have developed a next generation transcatheter Left Atrial Appendage (LAA) closure system.
What makes their solution stand out when compared to other LAA closure devices is twofold. First, the procedure itself is simpler and does not require many of the pre-procedure measurements or device orientation as other closure devices in the market. Secondly, the Appligator device leaves minimal foreign material in the heart post-procedure – only a suture is left behind, thereby reducing the risk of stroke to the patient and preventing blood clot leakage by achieving complete LAA closure post-procedure.
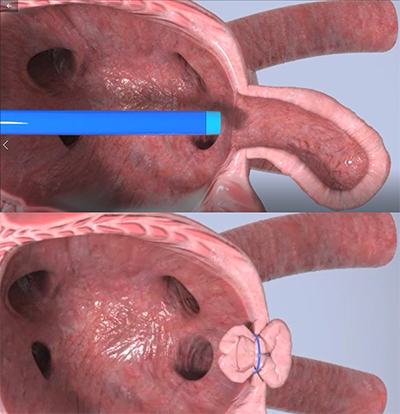
The Append Medical LAA Procedure
Rather than using a metal implant to close the opening, the Appligator manipulates the LAA tissue, using the LAA’s own tissue to seal it against itself, fastening it with a simple suture stitch.
Append are still some way away from commercialisation but the development of Appligator is continuing to accelerate thanks in no small part to their involvement in the MEDX programme. They’ve recently brought in Professor Horst Sievert, head of the Cardiovascular centre at Frankfurt as chairman of the scientific advisory board.
With a plan to start clinical trials in the next two years, Append are a company to keep an eye on.
Transverse Medical – Point-Guard
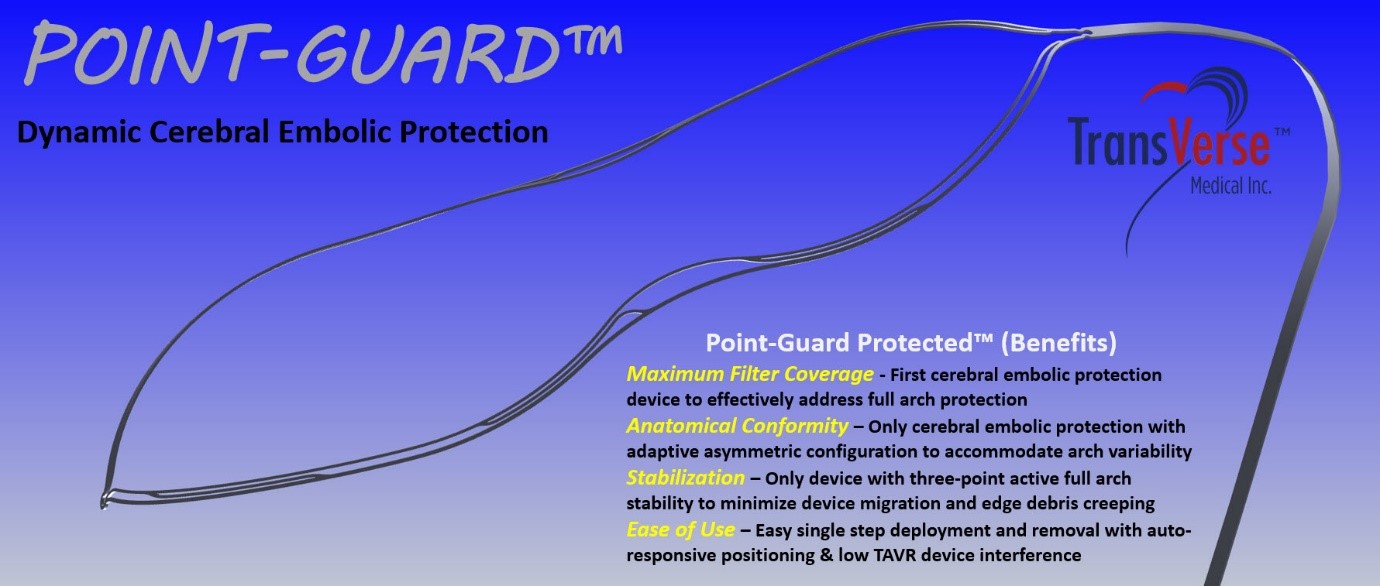
One of the most feared consequence of structural heart procedures like transcatheter aortic valve replacement (TAVR) or atrial fibrillation (AF) ablation is emboli reaching the brain and causing stroke or embolism. The Point-Guard™ solution from Transverse Medical is the first to provide a game changing innovative solution for this devastating complication and known risk of stroke through providing complete protection for the brain during TAVR procedures.
Point-Guard achieves this through addressing maximum filter coverage (Full Arch Protection), low TAVR device interference and facilitating ease of use for the operator. The Point Guard is the only TAVR compatible solution which can provide the brain with complete protection.
Point-Guard’s primary focus is on reducing the event of Stroke during cardiovascular procedures and has high confidence that their next generation Point-Guard (now in design freeze) will achieve this goal and will demonstrate clinical success in the “PASS” Feasibility study scheduled for early 2021. Additionally, the company’s future pipeline of products includes the Point-Guard Rebound™ for peripheral embolic protection during heart procedures and together with Point-Guard they will provide a synergistic and complete solution for total embolic protection during TAVR and other cardiovascular procedures.
CEO Eric Goslau told me that they’re at an extremely exciting stage in their development, with aims to gain CE mark and FDA approval within the next few years. Longer term, Transverse would like their solution to become the industry standard and play a major role in the global TAVR market.
Having gathered together a highly qualified team of execs, physicians and scientists led by Eric, they hope that Point-Guard will eliminate the risk for stroke through full arch and maximum filter protection during cardiac procedures.
Moray Medical – Moray Medical Robotic Catheter System
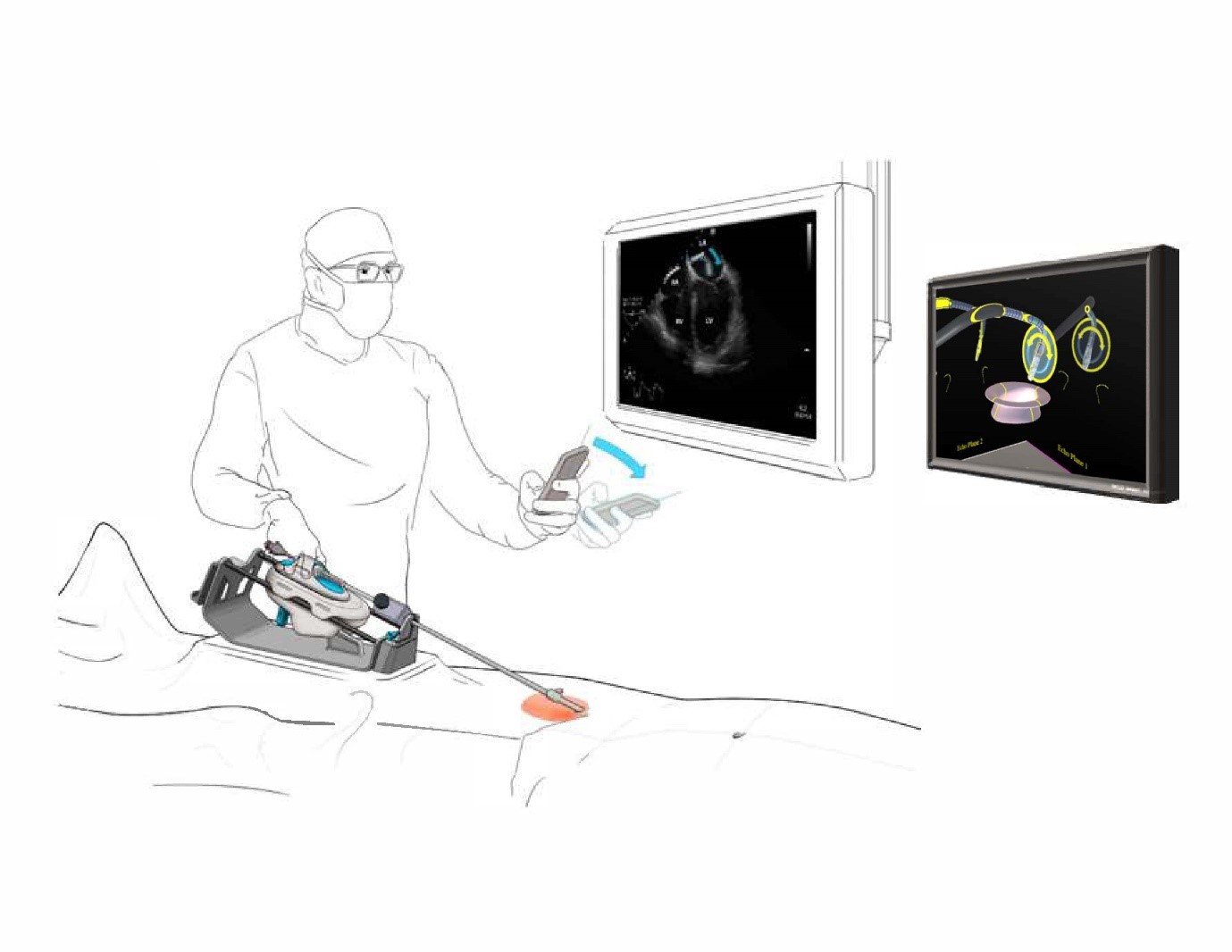
One of the most difficult elements of structural heart surgery is the delivery of devices and tools to the heart using a catheter. Founded in 2017 as Project Moray (named after the eel-like look of the device) Moray Medical have created a new, robotic method of delivery which will mean tens of thousands of interventional cardiologists will now have the ability to execute these highly complex procedures for millions of their patients.
Many structural heart procedures, such as mitral valve repair or replacement (TMVR), are outside the realm of expertise for most interventional cardiologists. That’s because accessing the necessary areas and positioning the replacement is an incredibly complex and technical procedure.
Moray’s device may help change that. By making delivery easier for cardiologists and driving cost per patient down with their simple system, these complex procedures could become accessible for many more interventional cardiologists more used to delivering stents and other more standard procedures.
They’ve made a robot which has less than 200 mechanical parts end to end, which is extremely unusual for the robotic medical device market. With leaders boasting companies like Intuitive and Accuray on their resumes, they also have the pedigree to back up the potential. So far Moray have managed to grow organically with their exceptional industry connections working in tandem with the innovative solution the product provides.
Moray are aiming to file for FDA approval in early 2021 with commercialisation expected to follow in 2024 or 2025.
CardioMech – MVRS

CardioMech are still in the early stages of development, with a feasibility study being planned for 2021. However, the technology has the potential to be a breakthrough device for those suffering from severe, symptomatic degenerative mitral regurgitation (DMR).
Their device is delivered to the heart with a catheter, rather than open heart surgery, and it anchors a prolapsed or flailing mitral valve leaflet to secure it in place, potentially reducing or eliminating mitral regurgitation. It is designed to be a straightforward and simple procedure, with repositionable and retrievable anchors that gives the physician more options when implanting the device.
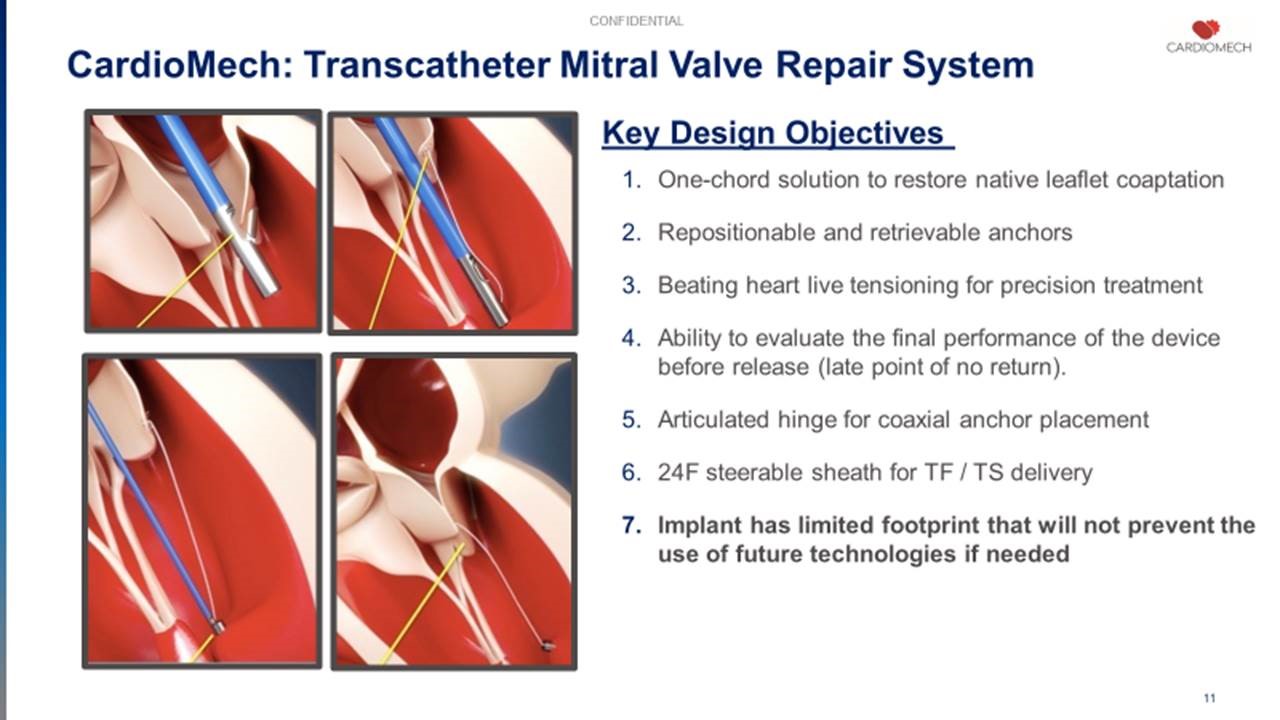
In the world of structural heart, where the tiniest of margins can make a huge difference, these factors could all combine to create a remarkable new therapy option for DMR patients.
When I spoke with their President and CEO Rick Nehm, he was the first to say that they have a long way to go to earn regulatory approval to treat patients, but CardioMech has the right team, the right partners, the right market and the right technology. You wouldn’t bet against them.
Thubrikar Aortic Valve Inc
Dr Mano Thubrikar has been an expert in the aortic valve for decades. So much so, he authored a book titled ‘The Aortic Valve’ back in 1990. Therefore, when he left his 30 year career as a scientist to pursue his mission of creating a super durable aortic valve that is more similar to a natural valve than anything else on the market, it’s worth taking notice.
In their design of the valve, Mano and his team have prioritised durability and mimicking the natural aortic valve so as to function as efficiently as possible in the patient. When we spoke, he told me that the early evidence of this working was positive.
Early trials in sheep has shown less calcification than in other surgical valves, the main cause of valve degradation. Thus far tests have indicated that the valve will continue functioning beyond 890 million cycles, the equivalent of over 24 years in vivo.
The valve can be delivered without the need for balloon expansion which can cause tissue damage. Instead, the Optimum TAV prioritises an optimized leaflet design of the valve and avoids suture holes in the leaflets, again contributing to durability for the patient. They have done a successful implant in one patient with excellent results.
The Optimum valve is expected to start European clinical trials in 2020, with commercialisation to follow a couple of years later. In a process that started over 10 years ago, this highly innovative approach to valve design based on decades of research and expertise could be a game changer for the aortic valve space.
I'd like to say a huge thank you to everyone who took the time out to speak to me about their businesses for this article. If these six structural heart businesses are anything to go by, it's safe to say that the future of the space is incredibly exciting.
Do you have anything you'd like to add? Any companies you’d like to discuss or that you think deserve attention in structural heart? If so, get in touch with me at james.christopher@medical-cm.com
Recommended.

The Future of Catheter Ablations with EP Frontiers CEO, Avi Fischer.
In this episode I talk to ex-physician gone start-up CEO, Avi Fischer, about the future of Catheter Ablations and how EP Frontiers is disrupting the industry. Click to listen.
.jpg)
Can PFA Revolutionise Cardiac Ablations?
With the limited data we have so far, PFA has proven to be safer than other ablation energies and to provide quicker recovery times. But is it ready to revolutionise cardiac ablations? To find out, I spoke to five leaders from start-ups in the space.

How to Take Your Medical Device to Market.
In this episode, I'm joined by Steven Haken and Deborah Rizzi from market access and reimbursement specialists Odelle Technology to discuss how to take a medical device to market.

How Much Value is in Your Data?
I caught up with the Head of Digital Health, Diagnostics & Monitoring at Biotronik, Ken Nelson, to talk about the increasing value of data within the space.
Comments.