

Innovative Spine & Orthopedic Implant Technologies to Watch.
Orthopedics isn’t necessarily a market commended for its digital innovation. Focused on the care of the musculoskeletal system, which interconnects muscles, bones, joints, ligaments, and tendons, the orthopedics sector has often been overlooked in the context of “medical disruptors”. However, given that orthopedic medical conditions are some of the biggest contributors to chronic pain in adults, I see this as a vast oversight.
For example, back pain. From 2019 up to 39% of US adults over 18 reported experiencing recent severe back pain (within three months). With more than half of adults in the United States suffering from back pain for over five years.
There’s no denying that this makes for a booming market, in response orthopedics has in fact seen impressive new technologies to match. In my role as a specialist consultant recruiting in this market, I'm always keeping my eyes peeled for new trends and products and have noted some true innovators in recent months.
With the Global Orthopedic Implants Market set to generate up to $68.80 Billion by 2030, let’s take a look at some of the products set to facilitate, and contribute to that growth.
Miach Orthopaedics
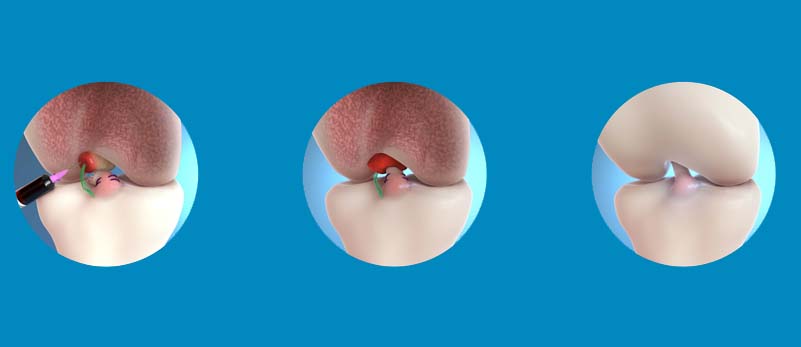
Dedicated to developing bio-engineered surgical implants for connective tissue restoration, Miach Orthopaedics Bridge-Enhanced ACL Restoration (BEAR®) Implant is a true market innovator. A product that represents a paradigm shift in the treatment of ACL tears from reconstruction to restoration.
Pioneered by Martha Murray, MD and founder of Miach Orthopaedics, at the Boston Children’s Hospital Department of Orthopaedic Surgery, the BEAR Implant represents many firsts for the industry. The first innovative treatment for ACL tears in decades with the potential to change the standard of care for ACL injuries, the first technology to clinically demonstrate restoration of the ACL, and the first of its kind to be cleared by the U.S. FDA through the De Novo Pathway.
The BEAR Implant is a decellularized, bovine-derived, type I collagen implant that resorbs within 8 weeks of implantation.
The rehabilitation protocol for the implant has been carefully designed to help protect the ACL during the healing process. Where a usual ACL reconstruction replaces the ACL with a graft, the BEAR implant restores the native ACL by bridging the gap between the torn ends.
When combined with an autologous blood clot the gap is temporarily bridged, protecting the clot from the harsh synovial environment. The BEAR implant then encourages the body’s natural healing response by supporting cell migration and proliferation. Within eight weeks, the implant is absorbed and replaced with native cells, collagen, and blood vessels. This new tissue then continues to strengthen over time with relevant physical therapy treatment.
Available for use on patients as young as 14, and as small as 45x25mm, this industry-leading invention has the potential to transform a previously debilitating injury into one with a standardized, achievable recovery time.
 Fuse Medical
Fuse Medical
Fuse Medical provides a broad portfolio of orthopedic implants including internal and external fixation products; upper and lower extremity plating and total joint reconstruction; soft tissue fixation and augmentation for sports medicine procedures; full spinal implants for trauma, degenerative disc disease, and deformity indications.
Fuse Medical’s broad portfolio of Orthopedic Implants and Biologics provide high-quality products to assist surgeons with positive patient outcomes and cost-effective solutions for customers. This offering is also set to expand after its recent merger agreement with BRM Extremities S.r.l. for the distribution of the latest advancements in lower extremities, namely the Silktoe Arthroplasty Implant.
Silktoe® offers severe arthritis patients an alternate offer to fusion, where bones are effectively welded to prevent joint pain.
Christopher Reeg, CEO at Fuse Medical, said:
"We believe the versatility of the device is superior to other toe arthroplasty products. We are excited to partner with BRM and look forward to expanding our distribution footprint with them in the US market.”
Intended for arthroplasty of the first metatarsophalangeal joint of the foot (or big toe, to those less medically trained like me), Silktoe represents a permanent implant. It can be used as a treatment for various severe forms of arthritis, such as hallux rigidus or hallux limitus, painful rheumatoid arthritis, hallux abducto valgus associated with arthritis, or unstable or painful joint from previous surgery.
According to Reeg’s comments in a recent release, Fuse is committed both to heroing innovative devices, such as this, as well as continuing to manufacture other popular implants. New technologies like Silktoe® demonstrate the ability to constantly create more effective solutions for today’s clinical challenges and utilize collaboration and innovation to assist with improving surgical outcomes.
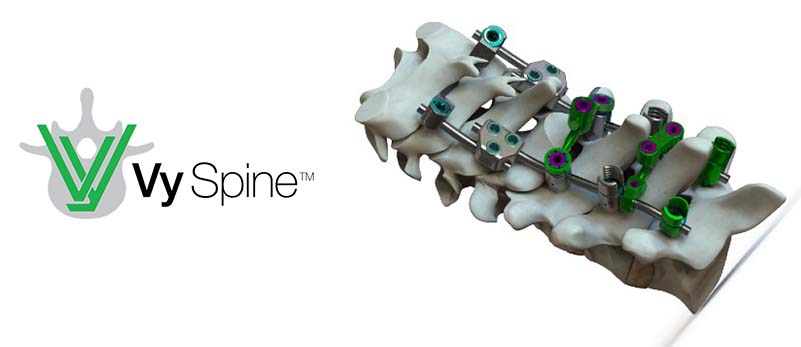 Vy Spine
Vy Spine
A leader in the orthopedic spine market, Vy Spine uses a mixture of material technologies to achieve superior results with its implants. This variety aims to optimize the offer of solutions to suit different environments.
The company has recently been granted 510(k) FDA clearance for its VySpan Posterior Cervical Thoracic System, meaning the device is safe and effective as, or substantially equivalent to, a legally marketed device.
The VySpan PCT System has a variety of different screw and hooks options, several transition rods, and revolutionary crosslink versatility, making the system arguably one of the most adaptable currently on the market.
The variety of vertebral anchor options, featuring hooks, standard thread screws, and smooth-shank screws, alongside the VySpan™ PCT System’s poly-axial heads, poly-axial reduction heads, fixed angle heads, and fixed angle reduction heads, means a myriad of unique and versatile cross-link options are available for ultimate strength, versatility, and stability. Head-to-head cross-links are available in double and single joint configurations, as well as traditional rod to rod cross-links, in double and single joint configurations if required.
This immense variety of implant options for the thoracic spine makes the system one of the most versatile and innovative to hit the market in some time and creates vast opportunities for clinicians and long-suffering orthopedic spine patients.
Tom McLeer, CEO of VySpine, said:
“The VySpan PCT system is the first of many highly differentiated systems being developed by VySpine. Using new materials and creative surgeon input, we are building exceptional quality, flexibility, and pricing into all our products. This is just the beginning of the exciting, innovative product launches scheduled for early 2022.”
Surgalign
In July 2020 Surgalign Spine Technologies was born a "new" company, despite having close to a 30-year heritage. Formerly RTI Surgical, a company with a sole focus on advancing spine surgery and elevating clinical outcomes, Surgalign rebranded with the same ethos and a whole new look.
Dubbing itself “the future in Spine”, this whole new look today helps launch Surgalign as a global medical technology company at the forefront of the market. A global leader in innovative spine solutions, its comprehensive line of spine products is backed by clinical research to reshape the standard of spine care solutions.
Deeply committed to new product innovation through partnerships with surgeons and industry experts, Surgalign has worked for the past five years to vastly increase the size of its business to meet the booming market needs. Its relentless focus on innovation and quality ensures patients receive the experience they deserve.
Most recently, the company welcomes its own FDA 510(k) clearance for its world-first AI lumbar spine procedure. The HOLO Portal system is the first-ever artificial intelligence (AI)-driven augmented reality (AR) guidance system for the spine, and the first clinical application of Surgalign’s HOLOTM AI digital health platform. The system promises a new wave of collaboration between clinician and patient through a technology-led system.
Professor Paul Lewicki, PhD, a pioneer in AI and predictive analytics, commented:
“I recognize the power associated with HOLO technology, specifically the machine learning and AI algorithms and their ability to revolutionize healthcare. We spent years developing machine learning based neural networks to teach the computer anatomy and address specific surgical needs. The result is displayed in 3D directly in the surgeon’s field of vision using the AR display, allowing for an elegant flow of information between the system and the physician.”
 SeaSpine
SeaSpine
Finally, SeaSpine is a global medical technology company focused on the design, development and commercialization of spinal surgical solutions. Its comprehensive portfolio of orthobiologics and spinal implants solutions facilitate neurosurgeons and orthopedic spine surgeons in performing fusion procedures on the lumbar, thoracic and cervical spine.
SeaSpine’s spinal implants portfolio consists of an extensive line of products to facilitate spinal fusion in degenerative, minimally invasive surgery (MIS), and complex spinal deformity procedures. Most recently adding Admiral ACP to its spine care offering.
Admiral ACP represents the next generation of anterior cervical plating and was designed to strike the optimal balance between strength, profile, and construct rigidity. The product is designed to work seamlessly in the surgeon’s hands to create a more efficient and consistent anterior cervical discectomy and fusion (ACDF) experience. The design focuses on differentiated plate features combined with step-eliminating innovative instrumentation to ensure the most efficient application for the clinician.
Dr. Don Park, VC of Quality and Safety for the Department of Orthopaedic Surgery at UCLA, said:
“Admiral is the most versatile and robust ACDF system I have ever used. The screws, which feature a thread form optimized specifically for high angulation trajectories, and the threaded driver, are game changers. Admiral allows me to tackle my most difficult cases with confidence. I am excited that, with this full commercial launch, the greater market will now have access to such a comprehensive system.”
Most notably, the Admiral ACP system includes 1-5 level plating options with multiple crossover sizes, enabling surgeons to intraoperatively address specific surgical needs. Designed for use as fixation for ACDF procedures, SeaSpine recommends use in conjunction with other interbody offerings, including the 3D-printed Waveform™ C and Shoreline® RT. Admiral ACP once again showcases a great example of SeaSpine’s commitment to market-leading spinal implant systems as a solution for both patients and professionals.
What’s next?
I am really excited to see the success of these technologies as they roll out and look forward to learning what other exciting opportunities are to come from this booming level of innovation. Will the wider orthopedics market be forced to speed up its adoption of these technologies to meet market needs? Or will the reality see these new products take some time to become accessible for all?
If you’d like to discuss my thoughts on the technologies or companies featured, or wider market trends, in some more detail, then drop me a message at sean.lewis@medical-cm.com.
To learn more about what we do at CM Medical, click here.
To read more from industry leaders and consultants in the orthopedics market, click here.
Want to collaborate with us?
Feature in podcasts, articles & videos.Charlton Morris is a Talent Solutions business who offer search, contract, volume and employer branding solutions to the medical markets.
Recommended.
Innovative Spine & Orthopedic Implant Technologies to Watch.
Orthopedics isn’t necessarily a market commended for its digital innovation, but these five companies are proving differently with their latest product launches. Click to read more.
What Innovations Could Shape the Future of Orthopaedics?
To achieve personalised care in orthopaedics, we need advances in AI, surgical robotics, augmented reality (AR) and more! But where will this innovation come from? Click to find out.
3 Technologies Revolutionising Spinal Surgery.
New innovations have continued to advance surgery – especially within the spinal space. I wanted to highlight the revolutionary technology that is driving this change and shaping the future of the space.

How to Take Your Medical Device to Market.
In this episode, I'm joined by Steven Haken and Deborah Rizzi from market access and reimbursement specialists Odelle Technology to discuss how to take a medical device to market.

Comments.